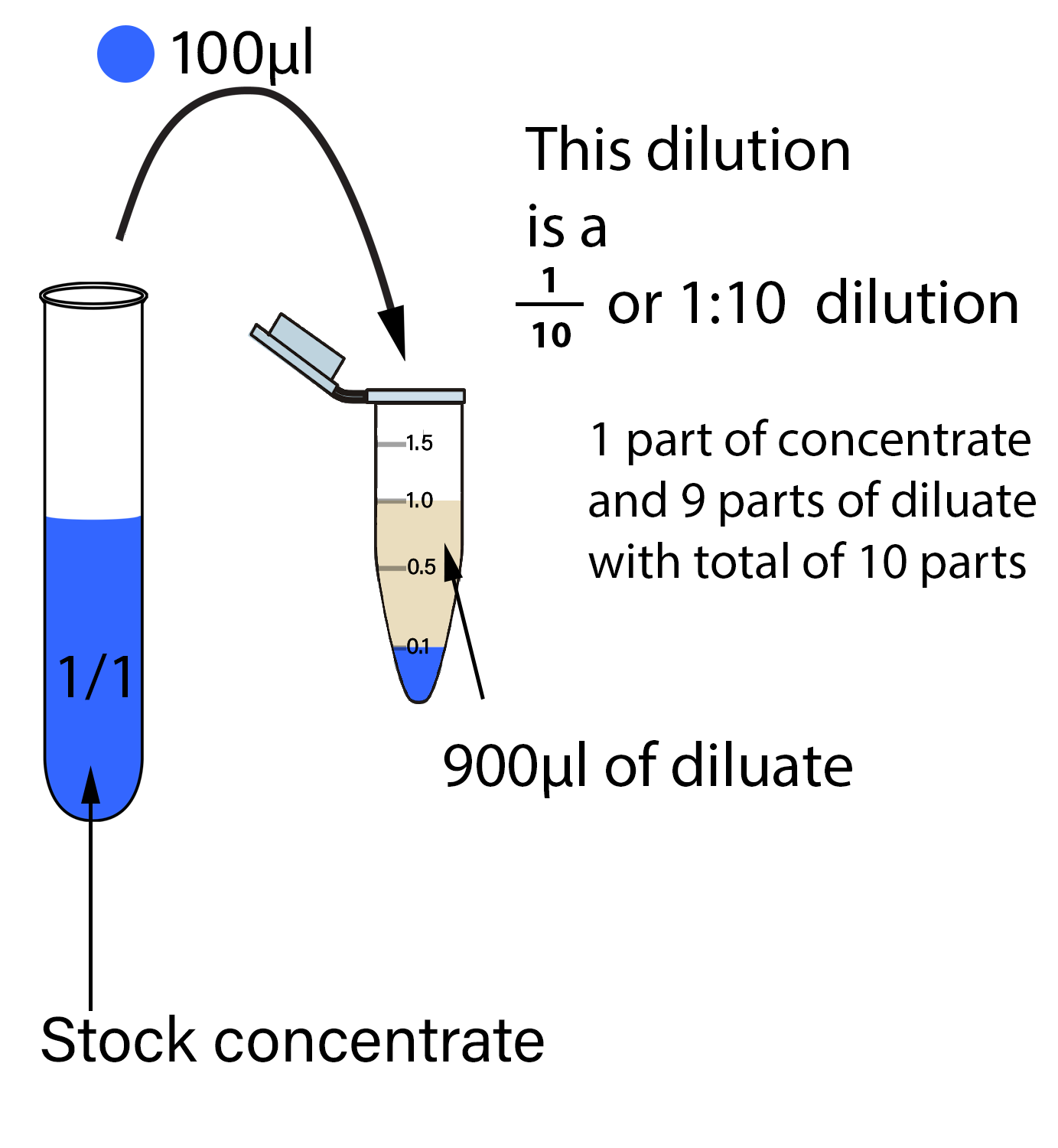
Serial Dilution Equation Examples . — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. — a serial dilution is the repeated. In this example, we are making 6. to perform a dilution you can always use the equation: — determine the number of dilutions, dilution factor (or range), and starting solution concentration. — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. (volume of solution)/(vol of solution + vol of solvent) since it is a two. A serial dilution is any dilution in which the concentration decreases by the same factor in each.
from giopkvexi.blob.core.windows.net
to perform a dilution you can always use the equation: In this example, we are making 6. — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. A serial dilution is any dilution in which the concentration decreases by the same factor in each. (volume of solution)/(vol of solution + vol of solvent) since it is a two. — a serial dilution is the repeated. — determine the number of dilutions, dilution factor (or range), and starting solution concentration.
Dilution Effect Definition at Robert blog
Serial Dilution Equation Examples to perform a dilution you can always use the equation: — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. A serial dilution is any dilution in which the concentration decreases by the same factor in each. — determine the number of dilutions, dilution factor (or range), and starting solution concentration. — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. to perform a dilution you can always use the equation: (volume of solution)/(vol of solution + vol of solvent) since it is a two. In this example, we are making 6. — a serial dilution is the repeated.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Serial Dilution Equation Examples A serial dilution is any dilution in which the concentration decreases by the same factor in each. In this example, we are making 6. to perform a dilution you can always use the equation: — a serial dilution is the repeated. — this article describes what a serial dilution is, provides examples of common applications, and explains. Serial Dilution Equation Examples.
From www.slideshare.net
Serial dilution Serial Dilution Equation Examples (volume of solution)/(vol of solution + vol of solvent) since it is a two. A serial dilution is any dilution in which the concentration decreases by the same factor in each. — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of. Serial Dilution Equation Examples.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Serial Dilution Equation Examples — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. (volume of solution)/(vol of solution + vol of solvent) since it is a two. A serial dilution is any dilution in which the concentration decreases by the same factor in. Serial Dilution Equation Examples.
From www.youtube.com
Microbiology Serial Dilution calculation YouTube Serial Dilution Equation Examples A serial dilution is any dilution in which the concentration decreases by the same factor in each. to perform a dilution you can always use the equation: In this example, we are making 6. — a serial dilution is the repeated. — serial dilution is the process of diluting a sample with a sterile diluent, which can. Serial Dilution Equation Examples.
From giobwgqpk.blob.core.windows.net
Dilution Formula Explained at Patti Tejada blog Serial Dilution Equation Examples to perform a dilution you can always use the equation: — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. In this example, we are making 6. (volume of solution)/(vol of solution + vol of solvent) since it is. Serial Dilution Equation Examples.
From educationhealey.z13.web.core.windows.net
How To Calculate Cfu/ml Serial Dilution Equation Examples In this example, we are making 6. to perform a dilution you can always use the equation: — determine the number of dilutions, dilution factor (or range), and starting solution concentration. — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a. Serial Dilution Equation Examples.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Serial Dilution Equation Examples — determine the number of dilutions, dilution factor (or range), and starting solution concentration. — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. — a serial dilution is the repeated. — serial dilution is the process of diluting a sample with a sterile diluent,. Serial Dilution Equation Examples.
From hxeysieuu.blob.core.windows.net
How To Make A 1 To 5 Dilution at Steve Greene blog Serial Dilution Equation Examples — a serial dilution is the repeated. to perform a dilution you can always use the equation: — determine the number of dilutions, dilution factor (or range), and starting solution concentration. — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. — serial dilution. Serial Dilution Equation Examples.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Serial Dilution Equation Examples — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. — a serial dilution is the repeated. to perform a dilution you can always use the equation: — serial dilution is the process of diluting a sample with a sterile diluent, which can be either. Serial Dilution Equation Examples.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Serial Dilution Equation Examples — a serial dilution is the repeated. — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. — determine the number of dilutions, dilution factor (or range), and starting solution concentration. to perform a dilution you can. Serial Dilution Equation Examples.
From www.pinterest.com.mx
Example of a Serial Dilution Medical laboratory science, Medical Serial Dilution Equation Examples — a serial dilution is the repeated. (volume of solution)/(vol of solution + vol of solvent) since it is a two. — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. — serial dilution is the process of diluting a sample with a sterile diluent, which. Serial Dilution Equation Examples.
From giowrfocz.blob.core.windows.net
Dilution Instructions at Dawn Morey blog Serial Dilution Equation Examples (volume of solution)/(vol of solution + vol of solvent) since it is a two. — a serial dilution is the repeated. In this example, we are making 6. to perform a dilution you can always use the equation: A serial dilution is any dilution in which the concentration decreases by the same factor in each. — this. Serial Dilution Equation Examples.
From giopkvexi.blob.core.windows.net
Dilution Effect Definition at Robert blog Serial Dilution Equation Examples In this example, we are making 6. — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. — determine the number of dilutions, dilution factor (or range), and starting solution concentration. — a serial dilution is the repeated.. Serial Dilution Equation Examples.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Serial Dilution Equation Examples — a serial dilution is the repeated. — determine the number of dilutions, dilution factor (or range), and starting solution concentration. In this example, we are making 6. — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. A serial dilution is any dilution in which. Serial Dilution Equation Examples.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Serial Dilution Equation Examples A serial dilution is any dilution in which the concentration decreases by the same factor in each. In this example, we are making 6. — a serial dilution is the repeated. — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. — serial dilution is the. Serial Dilution Equation Examples.
From www.chegg.com
Solved Calculating Standard Curves and Understanding Serial Serial Dilution Equation Examples — a serial dilution is the repeated. — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. In this example, we are making 6. (volume of solution)/(vol of solution + vol of solvent) since it is a two. . Serial Dilution Equation Examples.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Serial Dilution Equation Examples — a serial dilution is the repeated. — serial dilution is the process of diluting a sample with a sterile diluent, which can be either distilled water or 0.9 percent saline, in a series of standard. In this example, we are making 6. — this article describes what a serial dilution is, provides examples of common applications,. Serial Dilution Equation Examples.
From www.youtube.com
Chem143 Dilution Equation YouTube Serial Dilution Equation Examples — this article describes what a serial dilution is, provides examples of common applications, and explains how to perform a serial. In this example, we are making 6. (volume of solution)/(vol of solution + vol of solvent) since it is a two. — serial dilution is the process of diluting a sample with a sterile diluent, which can. Serial Dilution Equation Examples.